Solutions
Solution is a homogeneous mixture of two or more components.
If we have a solution of only two components, the solution is called binary solution.
If we have a solution of three components, the solution is called ternary solution.
And if we have a solution of four component that is called quaternary solution.
In this unit, we will discuss about binary Solutions only. In this type of solution, we have a solute and a solvent.
Solute: This component is present in small quantity.
Solvent: This component is present in large quantity.
Example: sugar is dissolved in water
Sugar is solute and water is solvent.
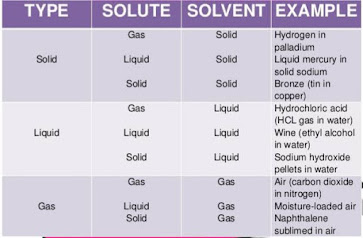
Types of solution:
Expressing concentration of solutions:
And if we have a solution of four component that is called quaternary solution.
In this unit, we will discuss about binary Solutions only. In this type of solution, we have a solute and a solvent.
Solute: This component is present in small quantity.
Solvent: This component is present in large quantity.
Solvent determines the physical state is which solution exists
Example: sugar is dissolved in water
Sugar is solute and water is solvent.
Types of solution:
Expressing concentration of solutions:
Composition of a solution can be described by expressing its concentration. The latter can be expressed either qualitatively or quantitatively.
Qualitatively, we can say that the solution is dilute or it is concentrated.
Quantitatively, we can define concentration in several ways.



Comments
Post a Comment